 LINKED PAPER
LINKED PAPER
Factors determining persistent asymmetry and egg shape in birds: A hypothesis. Deeming, D.C. 2022 Ibis. doi: 10.1111/ibi.13175 VIEW
Bird eggs come in a variety of shapes, from the extremely pointed, asymmetrical eggs of guillemots and wading birds through to the near spherical and symmetrical eggs of some owls and birds of prey (Fig. 1). This diversity has driven the long history of the study of egg shape, which mainly focussed on trying to produce mathematical equations that describe shape as accurately as possible. This mathematics are often complex (See Preston, 1953, Biggins et al., 2018) and not particularly intuitive to understand. I admit that, for many years, the maths put me off being interested in egg shape; the absence of a single variable that described egg shape has effectively limited the analysis of how, or why, an egg adopts a shape. This was a shame because these are interesting biological questions.
A few years back, however, I encountered geometric morphometrics that allowed me to quantify an egg’s shape in a relatively simple way. My initial study (Deeming & Ruta, 2014) explored variety in egg shape in oviparous vertebrates and analysis produced metrics that described whether an egg was spherical or elongated, and the degree of its asymmetry. The analysis showed that eggs assigned to Mesozoic birds were symmetrical and elongated and were different in shape to modern bird eggs (Deeming & Ruta, 2014). This same methodology allowed me to explore possible factors that affected degree of elongation or asymmetry (Deeming, 2018). The degree of elongation of an egg was related to absolute egg-mass, whereas the degree of asymmetry was related to the ratio of the mass of the egg relative to female body mass. It seemed that some other external variable was affecting asymmetry – perhaps it was something skeletal, like the pelvis that surrounds the oviduct?

Figure 1 Examples of egg shape variability in birds. The eggs (left to right) of the Common Guillemot (Uria aalge), the Red-breasted Merganser (Mergus serrator) and the Common Kestrel (Falco tinnunculus). Note that images are not to the same scale © D.C. Deeming.
There is a general association between elongated eggs and long, shallow pelves and rounder eggs with shorted more angled pelves but as I explored the relationship between pelvic shape and egg shape, nothing statistically significant emerged. A high-profile study by Stoddard et al. (2017) also explored factors determining egg shape and found that feather hand-wing index was significant but explained <5% of the variation in the data. The report generated lots of media attention (see Birkhead, 2018) but the biological relevance of the finding was disputed and debated (Birkhead et al., 2019; Stoddard et al., 2019). I struggled to see how a ratio of wing feathers can influence egg shape, which must be established in the oviduct.
Eggs form in the female’s oviduct; after ovulation the yolk moves down this elongated tube, driven by a peristaltic wave generated by muscles in the oviduct wall. First, the yolk enters the magnum where the albumen proteins are laid down around the yolk. The egg-mass (i.e., yolk and albumen) now enters the isthmus, which is much narrower than the magnum, where the fibrous shell membranes are deposited. The egg then moves on to enter the shell gland, where the calcitic shell is deposited. After this is completed shell accessory materials (e.g., pigment or a cuticle) is deposited and the egg is laid.
Interestingly, if you dissect an egg-mass out of the oviduct after it has passed through the isthmus, it is already egg-shaped despite lacking a hard shell. If you soak an egg in a weak acid to etch away the hard shell, decalcified eggs also retain their shape. The evidence suggest that shape is laid down in the isthmus but how this was achieved was not really been considered. Todd & Smart (1984) and Smart (1991) suggested that it was variation in differential pressure exerted by different parts of the oviduct wall that produced the variety of shapes. However, the oviduct wall is relatively flimsy, and the pressure would have to be consistent between eggs and birds because within a species’ egg shape is generally very similar. This all seemed rather complicated and importantly didn’t explain how an egg retains its shape.
My hypothesis attempts to explain how egg shape is both established and fixed in the oviduct (Deeming 2023). Firstly, I envisage that as the egg-mass moves from the magnum into the isthmus, the physical restriction imposed by the isthmus lumen restricts its movement down the oviduct. As the leading edge of the egg-mass enters the isthmus it is squeezed forcing the remainder of the egg-mass in the distal magnum to bulge outwards, resulting in an asymmetrical shape (Fig. 2A). I propose that the various egg shapes observed in birds are produced by the interaction between the size of the egg-mass relative to female body mass, and the degree of the restriction of the isthmus. Thus, a large egg-mass entering a narrow isthmus will produce a pointed egg shape as the large volume of egg-mass is forced through a narrow tube (Fig. 2A). However, if the egg-mass is small relative to body mass, and the isthmus lumen is wide, more of the egg-mass can enter the isthmus and the degree of asymmetry is low. If the isthmus does not impose any noticeable restriction on a relatively small egg you end up with a near spherical shape (Fig. 2B).
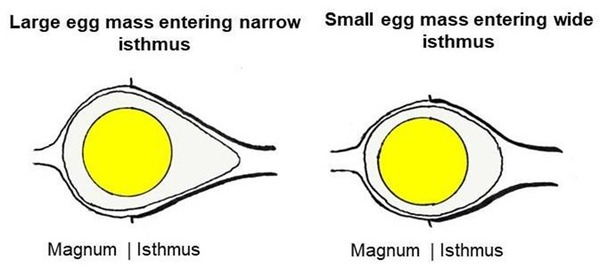
Figure 2 Diagrammatic illustration of the shape adopted by a large egg-mass entering a narrow isthmus (left), and by a small egg-mass entering a wide isthmus (right). The egg-mass is moving from left to right. From linked article (Deeming 2023).
The second part of the hypothesis relates to how egg shape is fixed. In the isthmus the shell membranes are laid down as a complex mesh of criss-crossing protein fibres, which each consist of a central core surrounded by a protein matrix (Lui et al., 2011). As they are deposited the matrix permanently sticks the fibres together. As the egg-mass moves through the isthmus, the shape that the leading part of egg-mass has adopted is fixed by the membranes. As the rest of the egg-mass follows through the isthmus the shape at the front end cannot be changed. Whatever shape the egg-mass has adopted in the isthmus will be retained by the fibrous mesh.
This hypothesis is the first that helps explain both how shape is formed and fixed in the oviduct. It can also apply to formation of shape in turtles and crocodiles, which produce symmetrical eggs that are spherical or elongated. All eggs in a clutch are formed at the same time along the length of the oviduct so in these reptiles there is no equivalent to the isthmus, so the egg-mass is not squeezed at any point during its formation and all eggs are symmetrical.
Of course, this hypothesis needs to be tested and it doesn’t explain why bird eggs are asymmetrical. One suggestion is that pointed eggs can more easily sit together in the nest which helps with the efficiency of contact incubation (Barta & Székely 1997; Birkhead et al., 2019) but how does this work for round eggs? Having provided a suggestion as to how egg shape is produced and fixed, we need to move forward to investigate why bird eggs exhibit variability in shape.
References
Barta, Z. & Székely, T. 1997. The optimal shape of avian eggs. Functional Ecology 11:656-662. VIEW
Biggins, J.D., Thompson, J.E. & Birkhead, T.R. 2018. Accurately quantifying the shape of birds’ eggs. Ecology and Evolution 8:9728-9738. VIEW
Birkhead, T.R. 2018. The shape of birds’ eggs. #theBOUblog VIEW
Birkhead, T.R., Thompson, J.E., Biggins, J.D. & Montgomerie, R. 2019. The evolution of egg shape in birds: selection during the incubation period. Ibis 161:605-618. VIEW
Deeming D.C. & Ruta, M. 2014. Egg shape changes at the theropod-bird transition, and a morphometric study of amniote eggs. Royal Society Open Science 1:140311. VIEW
Deeming D.C. 2018. Effect of composition on shape of bird eggs. Journal of Avian Biology 49:e01528. VIEW
Li, N., Niu, L.-N., Qi, Y.-P., Yiu, C.K.Y., Ryou, H., Arola, D.D., Chen, J.-H., Pashley, D.H. & Tay, F.R. 2011. Subtleties of biomineralisation revealed by manipulation of the eggshell membrane. Biomaterials 32:8743-8752. VIEW
Preston, F.W. 1953. The shapes of birds’ eggs. Auk 70:160-182. VIEW
Smart, I.H.M. 1991. Egg-shape in birds. In: Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles, Deeming, D.C. & Ferguson, M.W.J. (eds), pp. 101–116. Cambridge, UK: Cambridge University Press.
Stoddard, M.C., Yong, E.H., Akkaynak, D., Sheard, C., Tobias, J.A. & Mahadevan, L. 2017. Avian egg shape: form, function, and evolution. Science 356:1249-1254. VIEW
Stoddard, M.C., Sheard, C., Akkaynak, D., Yong, E.H., Mahadevan, L. & Tobias, J.A. 2019. Evolution of avian egg shape: underlying mechanisms and the importance of taxonomic scale. Ibis 161:922-925. VIEW
Todd, P.H. & Smart, I.H.M. 1984. The shape of birds’ eggs. Journal of Theoretical Biology 106:239-243. VIEW
Image credit
Top right: Pezibear CC0 Pixabay.
If you want to write about your research in #theBOUblog, then please see here.




