
LINKED PAPER
Causes of the recent decline of a Lesser Kestrel (Falco naumanni) population under an enhanced conservation scenario. Aparicio, J. M., Muñoz, A., Cordero, P. J., & Bonal, R. 2023. IBIS. DOI: 10.1111/ibi.13145. VIEW
Over the last century, Lesser Kestrel (Falco naumanni) populations have undergone dramatic changes. A sharp drop in numbers between the 1950s and 1990s led to the IUCN assessing the species as ‘Vulnerable’ in 1994, but population increases later resulted in a reclassification as ‘Least Concern’ from 2011 onwards (BirdLife International 2021). However, a recent census revealed a severe decline of 6% per year since 2012 in Spain, which hosts around 40% of the European breeding population (Bustamante et al. 2020). These recent declines occurred after the establishment of the European Union (EU) Natura 2000 network of protected areas, which was designed to safeguard species and habitats of biodiversity conservation interest, and are happening despite species-specific conservation measures such as captive breeding, reintroduction, and nestbox provision. Does this mean that these conservation actions aren’t working?
A recent study in Ibis examined Lesser Kestrel colonies in La Mancha, Spain, to identify any population changes, determine their causes, and establish if they are being addressed by current conservation actions.
Drivers of population declines
José Miguel Aparicio and colleagues collected data on colony size, land use, building conditions (i.e. nest-site availability), and density of Orthoptera (i.e. prey availability) from 12 La Mancha colonies in 2003 and 2021. They found that the study population had undergone a severe decline of 39.4% since 2003. As a population peak was estimated to have occurred in 2012, this indicates an annual decrease of 6% per year since the peak, matching previous findings for the whole of Spain (Bustamante et al. 2020).
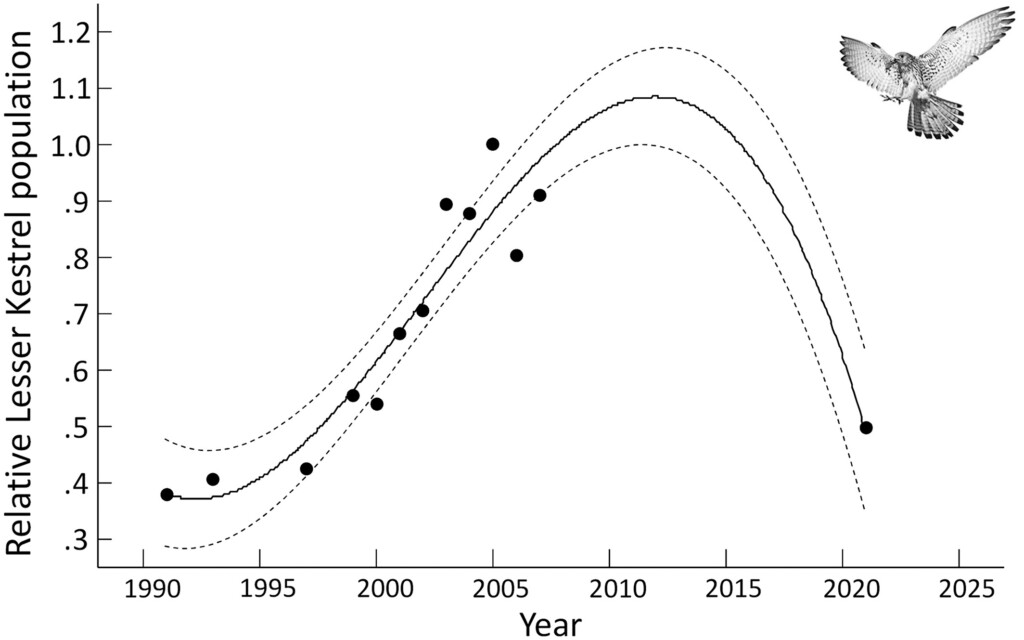
Figure 1. Evolution of the Lesser Kestrel population between 1991 and 2021. Dots indicate population size relative to the maximum registered population. The solid line indicates the population estimate by regression fit to a third-degree polynomial function and the dashed line the 95% confidence interval.
The researchers concluded that reduced availability of large Orthoptera, the main food for Lesser Kestrel nestlings in the study area, is the main cause of recent kestrel population declines. This reduced prey availability seems to have caused by land use changes, in particular the loss of the Lesser Kestrel’s preferred foraging habitats which contained the highest densities of large Orthoptera: herbaceous crops and pasture lands (Assandri et al. 2022). Results also indicated that limited nest-site availability could be a threat to Lesser Kestrels, with a a significant loss of nesting habitat from 2003-2021 due to the deterioration or demolition of the old buildings in which Lesser Kestrels breed. During the study, the researchers discovered a dramatic increase of rabbit (Oryctolagus cuniculus) populations, with rabbit abundance having a negative effect on kestrel colony size. While this may have been due to the effects of overgrazing by rabbits on vegetation and Orthoptera abundance, the researchers suspected it was more probably due to the process of hyperpredation caused by the increased rabbit density attracting birds of prey which may opportunistically prey on kestrels (Smith & Quin 1996).
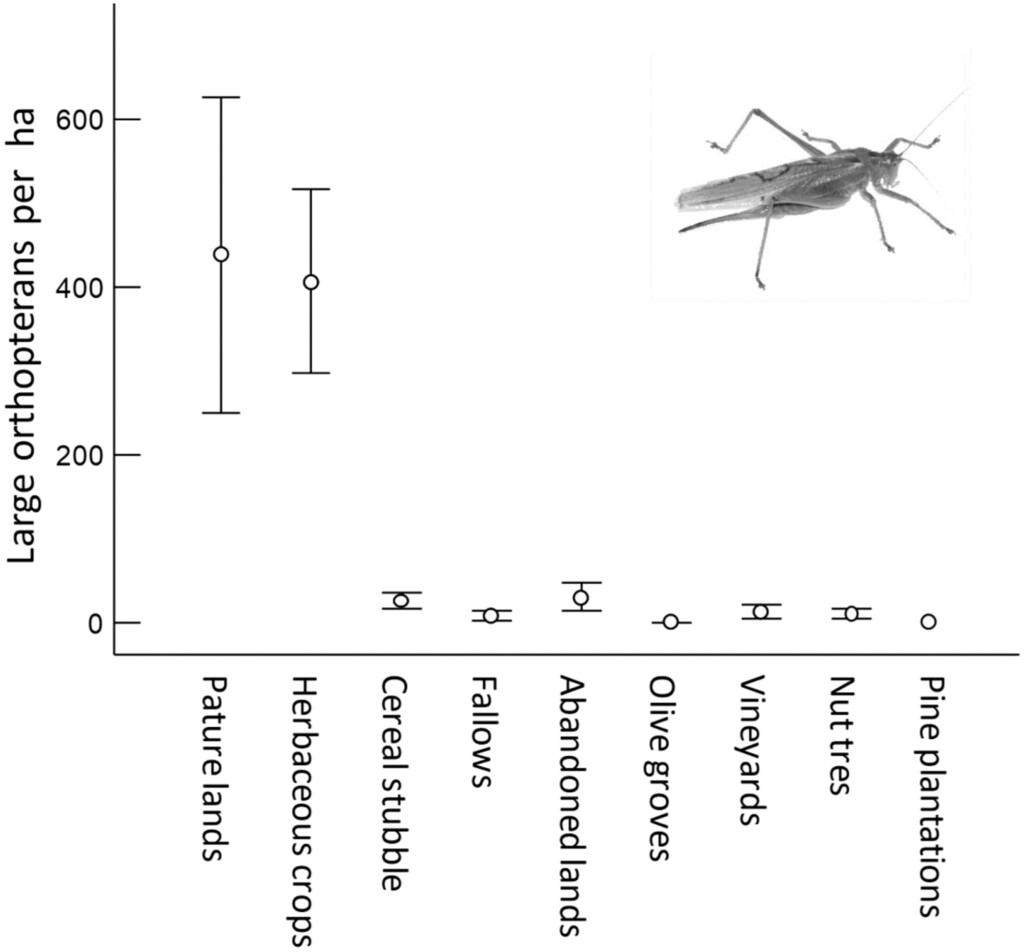
Figure 2. Mean (± se) density of large Orthoptera by land use in 2021.
Impact of conservation actions
While the inclusion of some areas within the Natura 2000 network appears to have slowed down land use changes and may have alleviated population declines, other actions such as the provision of artificial nestboxes had unclear effects, despite potentially being a way to mitigate the loss of nesting habitat. This may have been due to the population being limited by causes other than nest-site availability (Forero et al. 1996), or by nestboxes not being well designed or maintained. The nestbox entrance hole size was noted to be smaller than that of natural Lesser Kestrel nests, with a high level of occupation by Jackdaws (Corvus modedula). In fact, the increase in nestbox numbers was associated with an increase in the Jackdaw population, which could either compete with Lesser Kestrels or become allies against predators.
The researchers suggest future studies should examine whether the increase in nestboxes is facilitating the establishment of other species and analyse the consequences for Lesser Kestrels, as well as investigating the causal relationships behind the association between rabbit abundance and kestrel colony size.
References
Assandri, G., Cecere, J.G., Sarà, M., Catoni, C., De Pascalis, F., Morinay, J., Berlusconi, A., Cioccarelli, S., Mercogliano, A., Pazhera, A., Terras, A., Imperio, S., Morganti, M. & Rubolini, D. (2022). Context-dependent foraging habitat selection in a farmland raptor along an agricultural intensification gradient. Agriculture, Ecosystems & Environment 326: 107782. VIEW
BirdLife International (2021). Falco naumanni. The IUCN Red List of Threatened Species 2021: e.T22696357A205768513. VIEW
Bustamante, J., Molina, B. & del Moral, J.C. (2020). El cernícalo primilla en España, población reproductora en 2016–2018 y método de censo. Madrid: SEO/BirdLife. VIEW
Forero, M.G., Tella, J.L., Donfizar, J.A. & Hiraldo, F. (1996). Can interspecific competition and nest site availability explain the decrease of lesser kestrel Falco naumanni populations? Biological Conservation 78: 289–293. VIEW
Smith, A.P. & Quin, D.G. (1996). Patterns and causes of extinction and decline in Australian conilurine rodents. Biological Conservation 77: 243–267. VIEW
Image credits
Top right: Lesser Kestrel (Falco naumanni) | Sumeet Moghe | CC BY-SA 4.0 Wikimedia Commons
Blog posts express the views of the individual author(s) and not those of the BOU.
If you want to write about your research in #theBOUblog, then please see here




