Accentors have diverged and expanded rapidly across the Palearctic
LINKED PAPER
Explosive radiation and spatial expansion across the cold environments of the Old World in an avian family. Liu, B., Alström, P., Olsson, U., Fjeldså, J., Quan, Q., Roselaar, C.S., Saitoh, T., Yao, C.-t., Hao, Y., Wang, W., Qu, Y. & Lei, F. 2017. Ecology and Evolution. 7: 6346–6357. DOI: 10.1002/ece3.3136. VIEW
It is fascinating to note that some species have dramatically changed their ranges over a few decades. It is even more intriguing to try to understand which factors have shaped the current distributional patterns, and how these have varied through historical times. For example, some species or groups of closely related species are very patchily distributed across seemingly fairly homogeneous environments, suggesting that they were once more widespread, or that they have successfully colonized geographically disjunct areas. Climatic change, and resulting habitat transformations, have had profound impact on bird distributions.

The family Prunellidae (accentors) occurs entirely within the Palearctic region (Fig. 1), and accordingly has probably experienced pronounced range shifts during the Pleistocene climatic oscillations. Of the 13 species in this family, which are all placed in the genus Prunella, up to five are sympatric in the eastern Sino-Himalayan Mountains. Several others are allo-/parapatrically distributed, with a number of latitudinal and longitudinal range disjunctions. Some of these disjunctions are related to the patchiness of the preferred habitat, as most accentors breed near or above the upper tree line in mountains. The Dunnock P. modularis and Alpine Accentor P. collaris are exceptional in occurring from sea level to above the tree limit in different parts of their ranges. In fact, the latter species has the widest altitudinal distribution of any bird in the world, from sea level in coastal southeast Russia to nearly 8,000 m in the Himalayas (commonly to 5,600 m) (Hatchwell 2005).
In the present study, we estimated the phylogeny and divergence times for all of the world’s species of accentors based on multiple genetic loci, and analyzed morphometric divergence and biogeographical history.
Slow early divergences, later burst of speciations
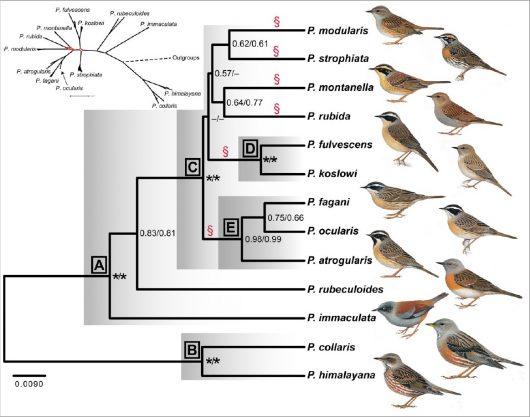
The phylogeny (Fig. 2) and chronogram (Fig. 3), in combination with a biogeographical analysis, suggest that the common ancestor of all accentors was found in the Sino-Himalayan Mountains or these mountains and Central Asia–Mongolia more than 9 million years ago. This split into two lineages, one resulting in a clade (B) with Alpine Accentor and Altai Accentor P. himalayana and another clade (A) comprising the other species. Within clade A, Maroon-backed Accentor P. immaculata and Robin Accentor P. rubeculoides branched off early, whereas the nine other species (clade C) underwent a rapid divergence during the mid-Pliocene to early Pleistocene. The relationships among the six primary lineages resulting from that differentiation (indicated by § in the figures) are essentially unresolved, probably because of the rapid radiation.

Range expansions during glacial periods
As most speciation events happened before the Pleistocene, the Pleistocene glaciations were not a major driving force in the evolution of accentors. However, the major climatic oscillations during that period may have substantially modified the distributions. We suggest that colonizations of new areas were facilitated during glacial periods, when suitable accentor habitats expanded away from the great Asian mountain ranges and plateaus across intervening low-elevation areas, followed by fragmentation of the suitable habitats during interglacial periods. This is supported by multiple late Pleistocene fossils of Alpine Accentor from the lowlands between mountainous regions in Europe (Tyrberg 1991). Although this scenario has been proposed by Tyrberg (1991), it has not been widely appreciated, and is contrary to the usual view that glacial periods lead mainly to fragmentation of populations.
Slow evolution of sympatry
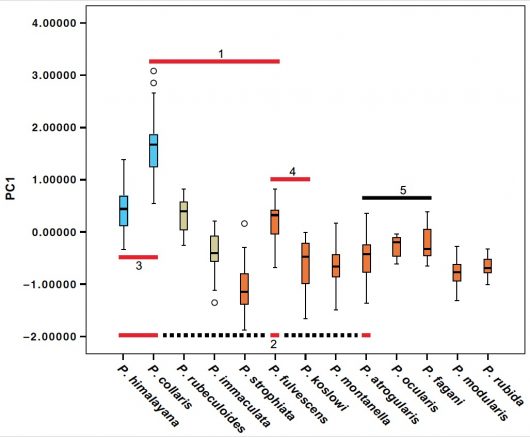
Our data suggest that sympatry increaed gradually with increasing time since divergence (Fig. 4). With one exception, species in clades younger than c. 3.7 million years are allopatric. We also found that species that are widely sympatric, including the most recently diverged sympatric sister species (Koslov’s Accentor P. koslowi – Brown Accentor P. fulvescens; 2.4 million years ago), are generally more divergent in size/structure than allo-/parapatric close relatives (Fig. 5). We also noted that all sympatrically breeding species are ecologically segregated, as suggested by differences in size/ structure and habitat choice. The distributional pattern and inferred ages suggest divergence in allopatry and substantial waiting time until secondary contact. The slow evolution of sympatry is likely due to competitive exclusion: only species that are sufficiently divergent in morphology and ecology are able to coexist.

Unresolved plumage evolution
Our phylogeny generally agrees poorly with the groups defined based on plumage similarity by Hatchwell (2005). However, all species with a prominent pale supercilium and contrasting dark crown and ear-coverts are found in the same clade (C), and, in agreement with Hatchwell (2005), the closely similar, and recently diverged, Black-throated Accentor P. atrogularis, Radde’s Accentor P. ocularis, and Yemen Accentor P. fagani form a strongly supported clade (E). Unfortunatley, the unresolved relationships within clade C precludes evaluation of whether the strong plumage similarity between the geographically widely separated Dunnock and Japanese Accentor P. rubida is due to convergence or close relationship.
References & further reading
Drovetski, S. V., Semenov, G., Drovetskaya, S. S., Fadeev, I. V., Red’kin, Y. A., & Voelker, G. 2013. Geographic mode of speciation in a mountain specialist avian family endemic to the Palearctic. Ecology and Evolution 3: 1518–1528. View
Hatchwell, B. J. 2005. Family Prunellidae (Accentors). In J. Del Hoyo, J. Elliott, & D. A. Christie (Eds.), Handbook of the Birds of the World, Vol. 10 (pp. 496–513). Lynx Edicions, Barcelona.
Tyrberg, T. 1991. Arctic, montane and steppe birds as glacial relicts in the West Palearctic. Ornithologische Verhandlungen 25: 29–49.
Image credit
Featured image: Koslov’s Accentor, Gobi Altai Mts., Mongolia, June 2016. © Per Alström
Blog posts express the views of the individual author(s) and not those of the BOU.
If you want to write about your research in #theBOUblog, then please see here.





