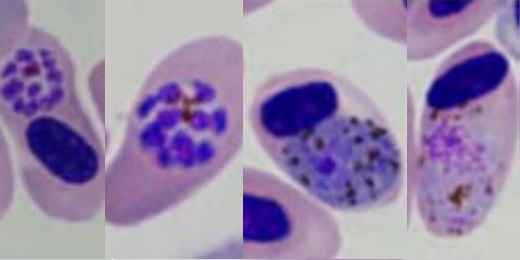
Slow embryo development is associated with increased resistance to blood parasites
LINKED PAPER
Duration of embryo development and the prevalence of haematozoan blood parasites in birds. Ricklefs, R. E., Ellis, V. A., Medeiros, M. C., Svensson-Coelho, M. 2018. The Auk. DOI: 10.1642/AUK-17-123.1. VIEW
The longer a bird incubates its eggs, the more likely those eggs will be lost to predation or bad weather (Ricklefs 1969). Because of this, one might expect natural selection to favor short incubation periods (i.e., time to hatching). However, incubation period varies more than two-fold among altricial bird species (i.e., those with dependent hatchlings) laying similar-sized eggs (Ricklefs 1993). Several hypotheses have been proposed to explain this apparent paradox (Ricklefs et al. 2017). For example, a longer incubation period implies slower embryo growth, which might in turn lead to higher quality offspring (Ricklefs 1993); accordingly, incubation period might be shaped by a trade-off between time-dependent egg mortality and offspring quality. Consistent with this hypothesis, Ricklefs (1992) found a negative relationship between incubation period and the prevalence of avian blood parasites among taxonomic families and sub-families of Nearctic and Neotropical birds. Ricklefs (1992) hypothesized that the negative relationship between incubation period and parasite prevalence was the result of increased B-cell diversity and, therefore, greater immunocompetence in birds with relatively long incubation periods.
The parasite data in Ricklefs’ (1992) analysis were derived from microscopic surveys of blood films and included information on the prevalence of several types of parasites. The most common parasites were haemosporidian parasites of the genera Plasmodium, Haemoproteus, and Leucocytozoon. Often referred to as avian malaria parasites, avian haemosporidians can be found infecting birds globally (Valkiunas 2005, Bensch et al. 2009) and can reduce host survival and lifetime reproductive success (e.g., Asghar et al. 2015).
Finding avian haemosporidian infections by microscopy, and distinguishing between the parasite genera, requires extensive training and experience (Fig. 1). In the time since Ricklefs (1992) published his study, PCR amplification and DNA sequencing have allowed researchers to detect and identify haemosporidians even at very low concentrations in avian blood. Studies comparing microscopy and molecular methods have shown that microscopy often fails to observe infections that molecular methods do detect (e.g., Ishtiaq et al. 2017; but see Valkiunas et al. 2008). It was therefore important to verify that the negative relationship between incubation period and parasite prevalence described in Ricklefs (1992) remained in analyses of parasite data generated with molecular methods.
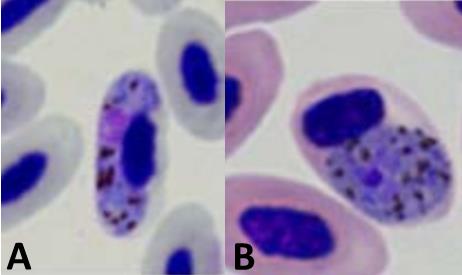
Figure 1 Stained blood smears showing Haemoproteus majoris (A) and Plasmodium relictum (B) infecting avian red blood cells. Photos from the P. B. Šivickis Laboratory of Parasitology in Vilnius
We assembled a dataset of Plasmodium and Haemoproteus prevalence obtained through molecular methods in Nearctic and Neotropical birds and compared it with the dataset obtained solely through microscopy by Ricklefs (1992). The molecular screening revealed nearly twice as many infections in the tropical samples as the microscopic methods, whereas estimates were more similar for the temperate samples. The molecular data supported the original finding of a negative relationship between incubation period and parasite prevalence (Fig. 2).

The new evidence we present supports earlier results and is consistent with the hypothesis that slower embryo development results in increased offspring quality (the latter expressed through resistance to parasite infection). However, more work is now needed to characterize the mechanism that generates this pattern. In particular, the genetic diversity of B-cells should be quantified in species that vary in the lengths of their incubation periods to test the hypothesis suggested by Ricklefs (1992). In the end, variation in incubation period length may be the result of several factors (Ricklefs et al. 2017), but the hypothesis of a trade-off between time-dependent egg mortality and offspring quality remains an intriguing explanation.
References
Asghar, M., Hasselquist, D., Hansson, B., Zehtindjiev, P., Westerdahl, H. & Bensch, S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347: 436-438. VIEW
Bensch, S., Hellgren, O. & Pérez-Tris, J. 2009. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Molecular Ecology Resources 9: 1353-1358. VIEW
Ishtiaq, F., Rao, M., Huang, X., & Bensch, S. 2017. Estimating prevalence of avian haemosporidians in natural populations: a comparative study on screening protocols. Parasites & Vectors 10: 127. VIEW
Ricklefs, R.E. 1969. An analysis of nesting mortality in birds. Smithsonian Contributions to Zoology 9: 1-48. VIEW
Ricklefs, R.E. 1992. Embryonic development period and the prevalence of avian blood parasites. Proceedings of the National Academy of Sciences USA 89: 472-4725. VIEW
Ricklefs, R.E. 1993. Sibling competition, hatching asynchrony, incubation period, and lifespan in altricial birds. Current Ornithology 11: 199-276. VIEW
Ricklefs, R.E., Austin, S.H. & Robinson, W.D. 2017. The adaptive significance of variation in avian incubation periods. The Auk 134: 542-550. VIEW
Ricklefs, R. E., Ellis, V. A., Medeiros, M. C. & Svensson-Coelho, M. 2018. Duration of embryo development and the prevalence of haematozoan blood parasites in birds. The Auk 135: 276-283. VIEW
Valkiūnas, G. 2005. Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton, FL, USA.
Valkiūnas, G., Iezhova, T.A., Križanauskienė, A., Palinauskas, V., Sehgal, R.N.M. & Bensch, S. 2008. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. Journal of Parasitology 94: 1395-1401. VIEW
Image credit
Featured image: Typical blood stages of Plasmodium relictum © P. B. Šivickis Laboratory of Parasitology
Blog posts express the views of the individual author(s) and not those of the BOU.
If you want to write about your research in #theBOUblog, then please see here.