 LINKED PAPER
LINKED PAPER
Colour of adult avian integumentary features reveals differences related to in ovo stressors. Krug-MacLeod, A.M., Eng, M.L., Cabezas, S., Marchant, T.A. & Morrissey, C.A. 2022 Ibis. doi: 10.1111/ibi.13150 VIEW
Adult birds’ skin and feather colour reflect stressors experienced before hatch
Can pre-hatch stressors have colour effects that carry into birds’ adult life? In essence, this question is a variation on the metaphorical question, “Can a leopard change its spots?” Differences in the colour of birds’ external features (integuments) can indicate degrees of individual quality as determined through genetics, self-maintenance, diet, or environmental influences (Lifshitz & St Clair 2016, Pérez-Rodríguez & Viñuela 2008). In vertebrates, carotenoid-based pigmentation – expressed as yellow, orange or red – is acquired either through direct dietary consumption (reflecting foraging ability) or through bioconversion of yellow carotenoids (reflecting antioxidant status) (Weaver et al. 2018a). Melanin-based pigmentation – expressed as brown or black – is produced directly and can vary with multiple markers of condition and stress (Minias et al. 2014). Both types of external pigmentation can be altered by behaviour or environment in the short term and can indicate individual quality in diverse bird species.
However, carotenoid and melanin integumentary pigmentation might also reflect the influence of in ovo stressors into adulthood. We tested this idea with captive adult Killdeer (Charadrius vociferus) that were retained following a study by Lunny et al. (2020) of hatchlings raised from eggs collected in the wild, then exposed to a combination of temperature conditions (low, optimal, or high) and polychlorinated biphenyl (PCB) 126 contamination (low, high, or control). We quantified colour using digital photography and measured physical features to investigate whether the colour of eye-rings and breast-bands and the morphology of the surviving yearling Killdeer varied according to the stressors experienced while in ovo.
Corticosterone levels obtained from newly hatched chick feathers collected during the initial study were evaluated as integrated signals of each individual’s adrenal response to the combined temperature and PCB stressors experienced in ovo (Bortolotti et al. 2008). The surviving birds were reared under identical food and environmental conditions and genetic effects were randomised and controlled in our analyses. Temperature treatment-associated variations in melanin and carotenoid-based ornament colour of breast-bands and eye-rings, biometrics and moult progression of adults suggested that carry-over effects of in ovo stressors might extend into adulthood.
Feather corticosterone at hatch was related to melanin-based (brown to black) breast-band colour and to higher incubation temperature. Hatchlings that had higher feather corticosterone levels had high melanin-pigmented, chromatically blacker breast-bands as adults. This association and the knowledge that mortality of chicks exposed to PCB-126 was exacerbated by high incubation temperatures (Lunny et al. 2020) led us to conclude that surviving birds were better able to cope with in ovo stress and therefore were of higher quality. This combination provided evidence that pre-hatch stressors might exert persistent effects on integumentary colour into adulthood.
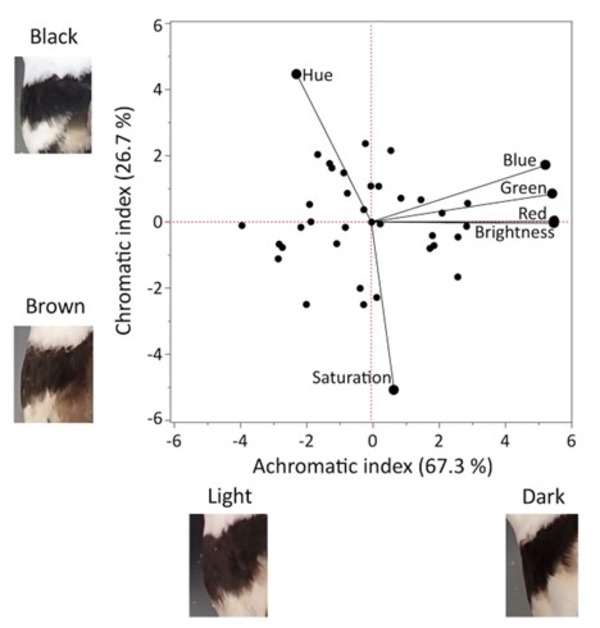
Figure 1 Principal components analysis (PCA) of Killdeer breast-band colour (n = 36) showing colour metrics with example images representing the extreme ends of the achromatic index (PC1) and chromatic index (PC2). Photographic metrics of the breast-band are obtained from analysis of breast-band digital photos (HSB and RGB values).
Chromatically yellower eye-rings in yearling birds were associated with low incubation temperature, while chromatically more orange eye-rings were associated with both optimal and high incubation temperatures. These eye-ring results suggest cold-stress may have a stronger effect on developmental outcomes than higher temperatures and that the effects of depleting carotenoids in ovo may last into adulthood (Koutsos et al. 2003, McGraw et al. 2005). Because eye-ring colour in several bird species is known to honestly signal sexual attractiveness (Kristiansen et al. 2006, Pérez-Rodríguez & Viñuela 2008), and mate choice and investment is affected by eye-ring colour (Alonso-Alvarez et al. 2012), we speculate that incubation temperatures may conceivably affect reproductive fitness in addition to early life survival.
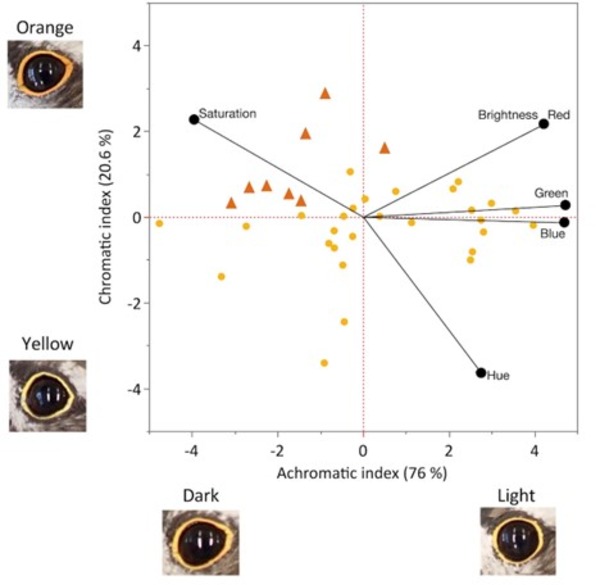
Figure 2 Principal components analysis of Killdeer photographic eye-ring colour (RGB and HSB values). (a) PC1 represents the achromatic index and PC2 represents the chromatic index. Symbols represent human observed eye-ring colours for each bird; orange triangles = observed orange (n = 8) and yellow circles = observed yellow (n = 28).
When evaluating standard adult bird biometrics in relation to integumentary colour quantification metrics, we found Killdeer with darker and blacker breast-band colour had longer headbill and culmen measurements (often associated with higher quality) but also shorter tarsus lengths. We also found a positive correlation between breast-band blackness and more advanced wing moult progression (indicated by a higher moult score). Birds of higher quality can be expected to moult earlier (Saino et al. 2013) because growth of new feathers is energetically expensive (Danner et al. 2015). Furthermore, newly moulted feathers are typically of higher quality (Delhey et al. 2010, Minias et al. 2015). The combined evidence of some larger biometrics and earlier moult in Killdeer with blacker breast-band feathers may reflect individual quality associated with more intense integumentary colour.
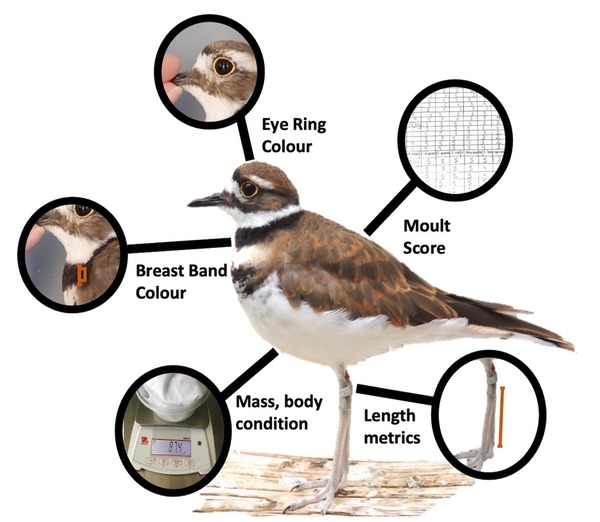
Figure 3 Key biometrics evaluated. Note: tarsus represents size, but culmen and bill were also measured.
The evidence indicating pre-hatch stressors may have carry-over effects on integumentary features in adult birds has implications for research and conservation. Our findings suggest that measurements of integumentary colour have potential value but should be interpreted cautiously when used to indicate current environmental conditions. If early life stressors can have carry-over effects on integumentary features in birds, then researchers should consider that colour signals may reflect long term effects of developmental stress and also reveal important differences in individual status caused by developmental conditions during embryonic life stages when proximate factors do not.
References
Alonso-Alvarez, C., Pérez-Rodríguez, L., Ferrero, M.E., García de-Blas, E., Casas, F. & Mougeot, F. 2012. Adjustment of female reproductive investment according to male carotenoid-based ornamentation in a gallinaceous bird. Behavioral Ecology and Sociobiology 66: 731-742. VIEW
Bortolotti, G., Marchant, T., Blas, J. & German, T. 2008. Corticosterone in feathers is a long‐term, integrated measure of avian stress physiology. Functional Ecology 22: 494-500 VIEW
Danner, R.M., Greenberg, R.S., Danner, J.E. & Walters, J.R. 2015. Winter food limits timing of pre‐alternate moult in a short‐distance migratory bird. Functional Ecology 29: 259-267 VIEW
Delhey, K., Burger, C., Fiedler, W., Peters, A. & Reby, D. 2010. Seasonal Changes in Colour: A
Comparison of Structural, Melanin- and Carotenoid-Based Plumage Colours (Plumage Colour Changes). PLoS One 5: e11582. VIEW
Koutsos, E.A., Clifford, A.J., Calvert, C.C. & Klasing, K.C. 2003. Maternal carotenoid status modifies the incorporation of dietary carotenoids into immune tissues of growing chickens (Gallus gallus domesticus). The Journal of Nutrition 133: 1132-1138 VIEW
Kristiansen, K.O., Bustnes, J.O., Folstad, I. & Helberg, M. 2006. Carotenoid coloration in great black-backed gull Larus marinus reflects individual quality. Journal of Avian Biology 37: 6/12 VIEW
Lifshitz, N. & St Clair, C.C. 2016. Coloured ornamental traits could be effective and non-invasive indicators of pollution exposure for wildlife. Conservation Physiology 4: cow028. VIEW
Lunny, E., Eng, M.L., Gurney, K.E.B. & Morrissey, C.A. 2020. Incubation temperature and PCB-126 exposure interactively impair shorebird embryo and post-hatch development. Environmental Research 188: 109779 VIEW
McGraw, K., Adkins-Regan, J. & Parker, E. 2005. Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colorful songbird. Naturwissenschaften 92: 375-380 VIEW
Minias, P., Kaczmarek, K., Wodarczyk, R. and Janiszewski, T. 2014. Melanin-based coloration covaries with fluctuating asymmetry, nutritional state and physiological stress response in common snipe. Journal of Avian Biology 45: 51-58. VIEW
Minias, P., Włodarczyk, R., Surmacki, A. & Iciek, T. 2015. Silver spoon effects on plumage quality in a passerine bird. Royal Society Open Science 2: 140459 VIEW
Pérez-Rodríguez, L. & Viñuela, J. 2008. Carotenoid-based bill and eye ring coloration as honest signals of condition: an experimental test in the red-legged partridge (Alectoris rufa). Naturwissenschaften 95: 821-830 VIEW
Saino, N., Romano, M., Caprioli, M., Lardelli, R., Micheloni, P., Scandolara, C., Rubolini, D. & Fasola, M. 2013. Molt, feather growth rate and body condition of male and female barn swallow. Journal of Ornithology 154: 537-547 VIEW
Image credit
Top right: Killdeer Charadrius vociferus © Alana Krug-MacLeod.
If you want to write about your research in #theBOUblog, then please see here.




