Are female African Penguins more vulnerable than males?
LINKED PAPER
Sex allocation and sex-specific parental investment in an endangered seabird. Spelt, A. and Pichegru, L. 2017. IBIS. DOI: 10.1111/ibi.12457. VIEW
African Penguins Speniscus demersus are endemic to Southern Africa and currently endangered after suffering from a drastic decrease of >90% in their population in the last century (Crawford et al. 2011). Previous research showed that the smaller female parent had a higher foraging effort during the chick rearing phase and suffered from a higher mortality at both juvenile and adult stage in comparison to males (Pichegru et al. 2013, Pichegru & Parsons 2014). Why is the mortality higher in female African Penguins? Are female African Penguins more sensitive to internal and/or external factors than males? Do they need special protection?
To answer some of these questions, I had the wonderful opportunity to spend a month with these special penguins in the austral summer 2015 on one of their colonies: Bird Island in Algoa Bay off Port Elizabeth, South Africa. Together with St Croix Island colony, Algoa Bay hosts half of the world population of African Penguins. My co-author Dr Lorien Pichegru has been studying these penguin populations since 2008 in order to find out whether competition with purse-seine fisheries contribute to the decline in African Penguin populations. In 2009, the South Africa’s Department of Environmental Affairs closed a radius of 20km around St. Croix as part of the ‘Island Closure Experiment’ in order to compare with Bird Island where the waters stayed open for fishing (Pichegru et al. 2010, 2012). Short term benefits of closures, such as reduced foraging effort, were observed rapidly but in the longer term 20km seemed to be too small to reverse penguin population decline, as average adult body mass and chick growth rates remained low.

African Penguins are long-lived species with bi-parental care and a clutch of two eggs. During the breeding season parents have to consider investment in their current offspring or in their own survival, with future reproductive prospects. Two different hypotheses are described to optimize this internal trade-off: the ‘fixed investment hypothesis’ and the ‘flexible investment hypothesis’ (Johnson et al. 1994). Fixed means that the parent provide constant investment regardless brood requirements, whereas flexible investments implies that parents adjust their investment according to internal and external factors. Long-lived species should favour their own survival and thus adopt a more fixed investment, however this differs per species but also within species between sexes.
Parents can also invest differently in their offspring according to the sex of their brood. This is known as the Trivers-Willard sex allocation theory, which predicts that parents should invest in the more beneficial sex when conditions are favourable (Trivers & Willard, 1973). Previous studies in seabirds have shown that the more costly sex is produced when food conditions were good, and when mate quality and female body conditions were high (Addison et al. 2008, Blanchard et al. 2007). This offspring sex ratio regulation can occur before egg formation (primary sex ratio adjustment) or at the time of hatching (secondary sex ratio adjustment) (Mayr 1939), but unequal parental investment in chicks of different sexes also lead to biased sex ratio at fledging, with repercussion on adult (or tertiary) sex ratio. The penguin populations in Algoa Bay showed a higher mortality of juvenile females compared to males, so we investigated the possible reasons behind this difference in mortality. We proposed two hypotheses in our study published in IBIS: (1) a higher investment towards male chicks could result in a higher survival chance of that sex into juvenile stage; and (2) a higher parental investment by the female during the breeding season could lead to higher energy expenditure and thus in lower survival. Additionally, we assess the possibility of male-biased offspring production which would in combination with higher female mortality lead to a male-biased adult sex ratio in African Penguins.

During our month on the island, the fieldwork team worked hard, even round the clock to record nest attendance surveys every 2 hours (yes, also at night) for 6 days from a total of 78 nests. We also collected adult and chick body measurements, and feathers (for DNA analyses) and filmed videos of feeding behaviour on the nest. Fieldwork on a deserted island (except for the penguins and gannets breeding there) can be challenging, especially when the wind direction turned unfavourable to field researchers, then granted by a ‘lovely’ smell. But being surrounded by the sounds and views of so many penguins was an amazing experience I will never forget. We sexed the adults by measuring their beak, a field-sexing method giving >90% assurance for African Penguins (Pichegru et al. 2013). However, the great challenge was to sex the African Penguin chicks as this had never been done before. After many long hours in the lab playing around with products concentrations, lots of sweat and stress, and painfully empty gels (where the penguin DNA is supposed to be visualized), we finally managed to successfully sex 118 penguin chicks.
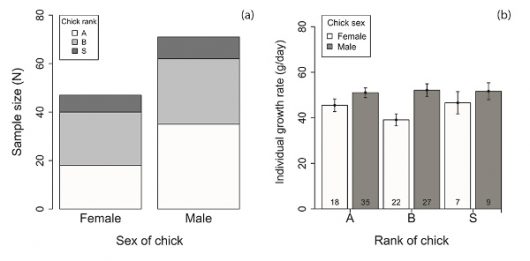
This data revealed for the first time a brood sex ratio biased towards males shortly after hatching. Male chicks also had higher growth rates and heavier fledging masses compared to females, suggesting a higher post-fledging survival. Moreover, female but not male parents had higher foraging effort and lower body condition with increasing number of male chicks in their brood. These results imply that breeding females showed flexibility in their parental strategy, but also that they have a higher investment cost in their current brood, which could contribute to the higher adult female mortality previously observed. This combination of male-biased chick production and higher female mortality, possibly at the juvenile’s stage from lower parental investment in female chicks, and at the adult’s stage from higher parental investment, may contribute to a biased adult sex ratio (ASR) in African Penguins.

Although ASR in the wild population of African Penguins is currently unknown, a male-skewed ASR could have serious consequences on population stability, as it may increase aggressive competition between males and reduce overall breeding success. If indeed female parents are more sensitive to external factors than males, current conservation efforts might need to focus particularly on females.
References and further reading
Addison, B., Kitaysky, A.S. & Hipfner, J.M. 2008. Sex allocation in a monomorphic seabird with a single-egg clutch: test of the environment, mate quality, and female condition hypotheses. Behaviour Ecology and Sociobiology 63: 135–141. VIEW
Blanchard, P., Hanuise, N., Dano, S. & Weimerskirch, H. 2007. Offspring sex ratio in relation to parental structural size and body condition in the long-lived Wandering Albatross (Diomedea exulans). Behaviour Ecology and Sociobiology 61: 767–773. VIEW
Crawford, R., Altwegg, R., Barham, B.J., Barham, P.J., Durant, J.M., Dyer, B.M., Geldenhuys, D., Makhado, A.B., Pichegru, L., Ryan, P.G. & Underhill, L.G. 2011. Collapse of South Africa’s penguins in the early 21st century. African Journal of Marine Science 33: 139–156. VIEW
Johnsen, I., Erikstad, K.E. & Stether, B. 1994. Regulation of parental investment in a long-lived seabird, the Puffin Fratercula arctica: an experiment. Oikos 71: 273–278. VIEW
Pichegru, L., Grémillet, D., Crawford, R. J. M., & Ryan, P. G. 2010. Marine no-take zone rapidly benefits endangered penguin. Biology Letters: rsbl20090913. VIEW
Pichegru, L., Ryan, P.G., Van Eeden, R., Reid, T., Gremillet, D. & Wanless, R. 2012. Industrial fishing, notake zones and endangered penguins. Biological Conservation 156: 117–125. VIEW
Pichegru, L., Cook, T., Handley, J., Voogt, N., Watermeyer, J., Nupen, L. & McQuaid, C.D. 2013. Sex-specific foraging behaviour and a field sexing technique for Endangered African penguins. Endanger. Species Res. 19: 255–264. VIEW
Trivers, R.L. & Willard, D.E. 1973. Natural selection of parental ability to vary the sex ratio of offspring natural selection of parental ability to vary the sex ratio of offspring. Science 179: 90–92. VIEW
To find more information about the African Penguin research in Algoa Bay please follow this link
Image credit
Featured image: African Penguin Speniscus demersus pair © Gwen Traisnel
If you want to write about your research in #theBOUblog, then please see here.





