LINKED PAPER
Signals of local adaptation across an environmental gradient among highly connected populations of the Dead Sea Sparrow Passer moabiticus in Israel. Cohen, T.M., Haran, R. & Dor, R. IBIS. DOI: 10.1111/ibi.12652. VIEW
Not much is known about the enigmatic, often elusive, Dead Sea Sparrow, Passer moabiticus. First described from the Dead Sea region, this small passerine (the smallest of its genus) is now known to occur in a highly disjointed range that spans from southwest Afghanistan to Cyprus in the west (Fig. 1a). It prefers semi-arid habitats with high temperatures adjacent to water resources, such as the Judean Desert and the Jordan Rift Valley in Israel, where it employs its skills as a gifted architect to build a large nest where the eggs, which are kept warm by the ambient temperature, don’t overheat. This expertise allows the parents to reduce incubation time and spend more time foraging for food.
 Figure 1 (a) Sampling locations of Dead Sea Sparrow populations in Israel. Gray squares represent locations where genetic samples were collected. (b) Breeding and non-breeding range of populations of the Dead Sea Sparrow in its complete range. Black circles represent locations from which morphological samples were available for the study. Range specification as follows: dark gray – Extant (breeding), medium gray – Extant (non-breeding), light gray – Extant (resident) (adapted from BirdLife International and Handbook of the Birds of the World (2016). Passer moabiticus. The IUCN Red List of Threatened Species. Version 2017-1). Click to view larger image
Figure 1 (a) Sampling locations of Dead Sea Sparrow populations in Israel. Gray squares represent locations where genetic samples were collected. (b) Breeding and non-breeding range of populations of the Dead Sea Sparrow in its complete range. Black circles represent locations from which morphological samples were available for the study. Range specification as follows: dark gray – Extant (breeding), medium gray – Extant (non-breeding), light gray – Extant (resident) (adapted from BirdLife International and Handbook of the Birds of the World (2016). Passer moabiticus. The IUCN Red List of Threatened Species. Version 2017-1). Click to view larger image
However, recent anthropogenic processes have led to a change in resource availability that allowed for expansion of the species distribution range and resulted in increase in abundance of the species in Israel, Jordan, Syria, Iraq, Turkey and Cyprus. This expansion resulted in the occurrence of the species in more humid, colder habitats, to the north and hotter habitats to the south. A climatic gradient can sometimes lead to changes in the characteristics of populations, and ultimately to local adaptation to the environmental conditions, but its effect is often constrained by gene flow among populations from different habitats. Therefore, we aimed to investigate the current status of the Israeli population of the Dead Sea Sparrow by exploring the morphological and genetic structure of the population. We expected that because of the small latitudinal gradient across Israel and the recent range expansion of the species that Dead Sea Sparrow populations would show no significant morphological adaptation to local environmental conditions, and that considerable gene flow would be taking place among populations.
We sampled a total of 61 birds in the breeding season in three localities along the rift valley during 2014-2015. We recorded wing length and weight, and extracted blood for genetic analysis. We complemented this dataset with morphometric data available from data deposited at the Israeli Bird Ringing Centre (IBRC) during the years 1981-2015 (n = 875), and data that we recorded from specimens at the British Museum Bird Collection at Tring (n = 51) and at The American Museum of Natural History in New York (n = 9). We used microsatellite genetic markers to account for genetic variation and determine the connectivity among the populations.
Our findings were surprising. On the one hand, our results indicated the existence of gene flow, suggesting high connectivity among populations. This suggests that individuals migrate locally between populations, resulting in a genetic structure of a metapopulation (Fig. 2). On the other hand, despite this recurrent gene flow, latitudinal variation in wing length (male and female) and body mass (male) was indicative of local adaptation across Israel, according to which individuals from the north had longer wings and were heavier than individuals from the south. This finding is in accordance with Bergmann’s rule, which stipulates that the body size of homoeothermic animals is larger in colder climates than in warmer ones, with the underlying mechanism pertaining to the need for optimal temperature control.
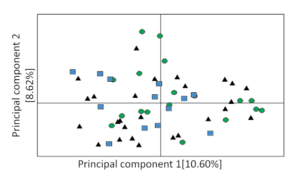
Figure 2 Principal Coordinate Analysis of Dead Sea Sparrow individuals from different localities as illustrated for nine microsatellite markers. Geographic regions are marked by colours and shapes as follows: blue square – Kfar Rupin, black triangle – Nahal Og, green circle – Hemar reservoir. Axes represent the percentage of total variation of the markers explained for each principal coordinate
Dead Sea Sparrows show strong breeding site fidelity, suggesting that despite seasonal movement, these individuals spend the better part of the year in the same area. This enables selection for phenotypic divergence related to the effect of temperature, as wing length (and body mass in males), and by proxy body size, increases with the decrease in average temperature. Alternatively, preliminary data collected from individuals captured during the breeding season and recaptured during wintering (R. Haran, unpublished data) revealed seasonal movement patterns from north to south during the winter, as average temperatures decrease. This suggests a possible role of wing length in local migration distances in allowing birds with longer wings to travel longer distances. However, a deeper understanding of the mechanism underpinning geographical variation in wing length requires additional exploration of the genetic and genomic architecture of the selected traits.
Despite learning about the structure of the Israeli population, the conservation status of the Dead Sea Sparrow is difficult to determine, partly because of its distributional range. The Israeli population examined here is considered a peripheral population, located at the southwestern border of the species’ range (Fig. 1b). As such, it may become even smaller and more fragmented, and gene flow to and from other populations may decrease. While gene flow can facilitate the emergence of adaptive alleles in a rapidly changing environment, it can also prevent peripheral populations from reaching the optimal trait phenotype through the introduction of poorly adapted immigrants. Moreover, if the rate of environmental change outpaces the extent of available genetic variation, including that which is introduced by migration, then the mean fitness and total density of the population are expected to drastically decline. Therefore, the Dead Sea Sparrow requires careful monitoring of its demographic and genetic variation, particularly in light of the morphological divergence found in the Israeli population. Moreover, additional research of the connectivity among populations across a larger part of the species’ range may help to shed light on the current status of the species from a global perspective.
Nominate this article for a BOU Science Communication Award.
References
Salewski, V., & Watt, C. 2017. Bergmann’s rule: a biophysiological rule examined in birds. Oikos 126 (2). VIEW
Lenormand, T. 2002. Gene flow and the limits to natural selection. Trends in Ecology & Evolution 17: 183–189. VIEW
García‐Ramos, G., & Kirkpatrick, M. 1997. Genetic models of adaptation and gene flow in peripheral populations. Evolution 51(1): 21-28. VIEW
Cohen, S. B., & Dor, R. 2018. Phenotypic divergence despite low genetic differentiation in house sparrow populations. Scientific Reports 8(1): 394. VIEW
Yom-Tov, Y., & Ar, A. 1980. On the breeding ecology of the Dead Sea Sparrow, Passer moabiticus. Israel Journal of Zoology 29(4): 171-187. VIEW
Image credit
Featured image: Dead Sea Sparrow Passer moabiticus © Roi Dor




