 LINKED PAPER
LINKED PAPER
Low genetic diversity and high gene flow in the Aquatic Warbler (Acrocephalus paludicola), a threatened marshland songbird with a fragmented breeding range. Kubacka J., Dubiec A., Korb J., Salewski V., Dyrcz A., Foucher J., Giessing B., Leisler B., Schulze-Hagen K., Wink M & Panagiotopoulou H. 2023 Ibis. doi: 10.1111/ibi.13250 VIEW
The Aquatic Warbler (Acrocephalus paludicola) is one of the rarest European songbirds, listed as vulnerable to extinction by IUCN (BirdLife International 2022). It is a migratory bird wintering south of the Sahara, specialised to breed in lowland open marshland – fens. Fens are grassland-type wetlands low in nutrients and typically dominated by sedges (Fig. 1), which formed thousands of years ago, mainly in river valleys. For centuries, fens have been lost and fragmented due to peat extraction, river regulation, drainage for agricultural use, excess of nutrients and abandonment of farming (Tanneberger & Kubacka 2018). Following the loss of breeding habitats, the Aquatic Warbler, once widespread in temperate Europe, suffered a steep decline. Only between the 1950s and 1980s the population shrank by 95% (Briedis & Keiss 2016). Today, the breeding range of the Aquatic Warbler is constrained to east-central Europe (Belarus, Ukraine, Poland and Lithuania), which holds c. 98% of the total population. As of the 1990s, the rate of decline has decelerated but the global population is 18,000-29,000 mature individuals, about three orders of magnitude less than in other common Acrocephalids such as the Sedge Warbler (A. schoenobaenus) (BirdLife International 2022).

Figure 1 A fen mire, breeding habitat of the Aquatic Warbler © Szczepan Skibicki.
Breeding habitat loss can lead to fragmentation of populations. This can slow down exchange of individuals between subpopulations (genetic isolation), induce loss of genetic diversity if a subpopulation is small (genetic drift), and enhance breeding of more related individuals (causing inbreeding depression). All of these processes negatively affect population growth and increase extinction risk, especially in habitat specialists (Pflüger et al. 2019). For this reason, studying population genetics is vital for effective species conservation. It helps indicate population management units, identify areas where habitat should be restored to reconnect isolated populations, and assess extinction risk more precisely (Holderegger et al. 2019).
In our recent study, we measured the genetic health of a large fraction of the Aquatic Warbler population. We used genetic markers (11 microsatellites) determined from blood samples recently collected in breeding areas (in 2014; contemporary) and about two decades earlier (in 1990-1998; historical). The contemporary samples were collected about 10-15 years after launching conservation actions to improve the breeding habitat, such as mowing to reduce overgrowth by reeds and bushes. Aquatic Warblers were sampled from about one quarter of their complete breeding range, in two subpopulations: Biebrza Valley and eastern Polesie in Poland (Fig. 2). We determined genetic diversity, expansions and reductions of population size, genetic structuring and exchange of individuals between subpopulations. We then compared these metrics both in space and in time.
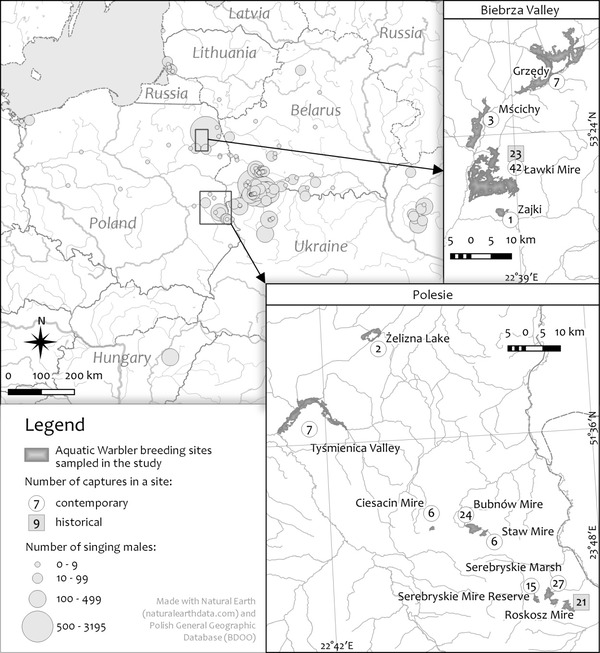
Figure 2 The sampled breeding sites and the breeding range of the Aquatic Warbler. Grey circles denote numbers of singing males as estimated between 2007 and 2014 © Kubacka et al. 2023.
The Aquatic Warblers from the studied subpopulations had low genetic diversity, which did not change over the study period. The expected heterozygosity (0.44-0.48) was comparable to that of endangered avian species (Evans & Sheldon 2008). We also found a moderate effective population size of approximately 200 individuals. This is regarded to be sufficient to protect populations from inbreeding depression, but not to retain their evolutionary potential in the long term (Frankham 2014). We detected a genetic bottleneck in the contemporary Biebrza Valley subpopulation, and (less certainly) in both historical and contemporary Polesie. Overall, these results show that the Aquatic Warbler harbours low genetic diversity, which is likely attributable to the decline it has experienced.
When we looked at the extent of population isolation, however, we did not find the Biebrza Valley and eastern Polesie to be genetically differentiated, in any of the two timepoints. Genetic clustering did not separate these two subpopulations, either. The two regions are separated by habitat unsuitable for the species and are about 250 km apart (Fig. 2). This means that Aquatic Warblers are able to move between and maintain efficient gene flow between distant breeding patches. Since the return rate of adult birds, at least males, is high (Tanneberger & Kubacka 2018), one mechanism of the high gene flow could be broad post-natal dispersal of young birds returning from wintering quarters.
Across time, we detected genetic differentiation and separate genetic clustering. It is likely, then, that allele frequencies between the historical and contemporary samples had changed. This could have been caused by genetic drift, or by mass immigration, for example if Aquatic Warblers were attracted to improved and restored habitats, or due to catastrophic events such as droughts, fires and floods. Factors shaping gene flow in the Aquatic Warbler should definitely be studied further.
Because the Aquatic Warbler breeds in a restricted geographic range (Fig. 2), and we studied about one quarter of it, our findings signal a warning that not only the sampled subpopulations, but the global population might be genetically impoverished. However, our results point to remarkably high genetic connectivity between distant locations. The ability to recolonise restored breeding sites and genetic resilience to habitat fragmentation appear high in the Aquatic Warbler. Taken together, our results provide a genetic argument to prevent further habitat loss and create stepping-stone breeding habitats between Aquatic Warbler subpopulations.
References
BirdLife International. 2022. Acrocephalus paludicola. The IUCN Red List of Threatened Species 2022: e.T22714696A176687364. Accessed on 25 July 2023. VIEW
Briedis, M. & Keiss, O. 2016. Extracting historical population trends using archival ringing data–an example: the globally threatened aquatic warbler. Journal of Ornithology 157:419-425. VIEW
Evans, S.R. & Sheldon, B.C. 2008. Interspecific patterns of genetic diversity in birds: correlations with extinction risk. Conservation Biology 22:1016-1025. VIEW
Frankham, R., Bradshaw, C.J.A. & Brook, B.W. 2014. Genetics in conservation management: Revised recommendations for the 50/500 rules, red list criteria and population viability analyses. Biological Conservation 170:56-63. VIEW
Holderegger, R., Balkenhol, N., Bolliger, J., Engler, J.O., Gugerli, F., Hochkirch, A., Nowak, C., Segelbacher, G., Widmer, A. & Zachos, F.E. 2019. Conservation genetics: linking science with practice. Molecular Ecology 28:3848-3856. VIEW
Pflüger, F.J., Signer, J. & Balkenhol, N. 2019. Habitat loss causes non-linear genetic erosion in specialist species. Global Ecology and Conservation 17:e00507. VIEW
Tanneberger, F. & Kubacka, J. 2018. The Aquatic Warbler Conservation Handbook. Potsdam: Brandenburg State Office for Environment (LfU). VIEW
Image credit
Top right: A male Aquatic Warbler © Justyna Kubacka.
If you want to write about your research in #theBOUblog, then please see here.





