 LINKED PAPER
LINKED PAPER
Sex-condition interaction affects Greater Flamingo colouration. Amat, J. A., Garrido, A., Rendón-Martos, M., Portavia, F., & Rendón, M. A. 2022 Ardeola. doi: 10.13157/arla.69.2.2022.ra3 VIEW
In species that inhabit unpredictable environments, the use of cosmetic colouration may signal dynamic changes in the physiological condition of individuals over short-term periods. In Greater Flamingos (Phoenicopterus roseus), dynamic colouration has been linked to the ability to provide parental care. But, how does the interaction of sex and habitat quality affect the body condition and plumage colouration of flamingos throughout the year?
Visual signals are used by animals to communicate to rivals and potential partners about their ability to obtain resources and, therefore, their physiological condition. Plumage colour depends on the combination of both pigments and light refraction due to the structure of feathers. Two main groups of pigments confer non-structural colouration to the plumage of birds: melanins and carotenoids. Melanins provide strengthening to the feathers, photoprotection and thermoregulation. Carotenoid pigments produce bright yellow, orange and red colouration. Unlike melanin, carotenoids cannot be synthesised by animals and must be obtained exclusively from the diet. Because of this, carotenoid-based pigmentation would reflect the nutritional condition of individuals.
Plumage colouration has been traditionally perceived as a static trait that may not adequately indicate individual condition throughout the year, as pigments are incorporated into feathers during moult. However, the expression of plumage can be tuned independently from moulting through feather degradation (e.g., differential abrasion in House Sparrow [Passer domesticus]), coverable colour patches (e.g., colour badges in Red-winged Blackbirds [Agelaius phoeniceus]), and cosmetic colouration of the feathers by using substances obtained from the environment (e.g., use of iron oxide in Bearded Vulture [Gypaetus barbatus]) or produced by the birds themselves (e.g., application of the secretions in Greater Flamingos), making plumage colouration a dynamic signal.
Greater Flamingos can modify their external appearance independently from moulting by applying their own make-up over the plumage. The secretions of the uropygial gland contain higher concentrations of carotenoids during periods when flamingos exhibit more colourful plumage. Furthermore, flamingos transfer pigments from the uropygial gland more frequently to the plumage during periods when the plumage is most colourful. Because the colour fades if no make-up is applied, the flamingos have to frequently apply the cosmetic over their feathers to be colourful. As a result of both higher pigment concentration and greater frequency of transfer to feathers, Greater Flamingos can modify the appearance of the upper back, chest, and neck plumage making it redder. This flexible application of make-up may be used by Greater Flamingos to signal the ability of individuals to track unpredictable environmental changes in wetlands inhabited by this species, which must be essential to signal the parental capability during the displaying periods, and likely the status during agonistic interactions.

Figure 1 A group of Greater Flamingos displaying. The image shows differences in colouration between individuals. The upper back, chest, and neck may be redder as a consequence of pigment application by rubbing their cheeks on the uropygial gland and thus transferring the pigments over the plumage like make-up © Manuel Díaz Zurita.
In our new study, published recently in Ardeola, we examined the relationships between plumage colouration, sex, foraging site, and body condition in Greater Flamingos in Southern Spain. We hypothesised that redder individuals might be perceived as dominants by paler ones, which would allow them to access better foraging resources and, therefore, show a better body condition than less colourful individuals. Furthermore, we tested if the relationship between plumage colouration and body condition was site-related. Finally, because females appear to be more energetically constrained than males, this study also tested whether it should be more important for females than males to signal their ability to obtain resources, and thus the relationship between colour and body condition should also be more apparent in females than in males.
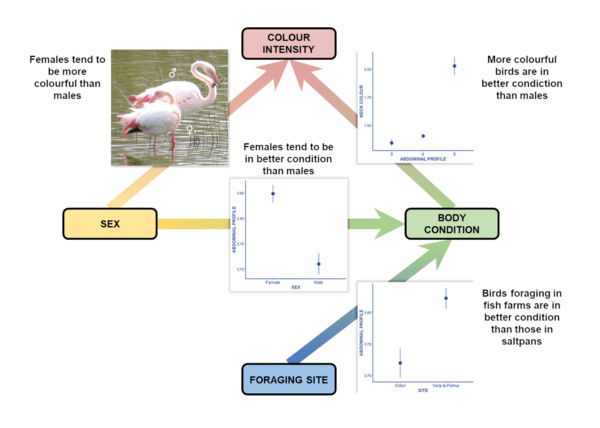
Figure 2 Diagram of relationships between plumage colour, body condition, sex, and foraging site in two wetlands in Southern Spain for a whole year © Manuel Díaz Zurita.
The results of our study show that birds in better body condition (more prominent abdominal profiles) tended to occupy more productive fish farms, while individuals in poorer condition fed in saltpans. In addition, female Greater Flamingos tended to be both more colourful and in better body condition than males. But, why do females need to signal their body condition more clearly than males? This may be because females are more physiologically constrained than males during breeding, and so females may limit the breeding success more than males. Females are smaller than males and, in addition to bearing the cost of egg production, they experience fasting periods during incubation just like males. Moreover, the chick provisioning rates of females are higher than those of males. As a consequence, reproductive costs may be higher for females, as incubation abandonment rates and the time needed to recover their body condition after each provisioning episode tend to be greater in females. Thus, the plumage colouration in the Greater Flamingo may act as an honest condition-dependent signal related to the parental effort.
Finally, our study also provides evidence towards a direct link between body condition and plumage colouration in both males and females, suggesting that the intensity of colour exhibited by flamingos may be an honest signal not only during the period of pair bonding but also at other times of the year when agonistic interactions can occur in the feeding areas.
References
Amat, J.A. & Rendón, M.A. 2017. Plumage colouration and its significance. In, M.J. Anderson (Ed.): Flamingos: Behavior, Biology, and Relationship with Humans, pp. 77-95. Nova Publishers, New York. VIEW
Amat, J.A., Rendón, M.A., Garrido-Fernández, J., Garrido, A., Rendón-Martos, M. & Pérez-Gálvez, A. 2011. Greater Flamingos Phoenicopterus roseus use uropygial secretions as make-up. Behavioral Ecology and Sociobiology 65: 665-673. VIEW
Amat, J.A., Garrido, A., Portavia, F., RendónMartos, M., Pérez-Gálvez, A., Garrido-Fernández, J., Gómez, J., Béchet, A. & Rendón, M.A. 2018. Dynamic signalling using cosmetics may explain the reversed sexual dichromatism in the monogamous Greater Flamingo. Behavioral Ecology and Sociobiology 72: 135 VIEW
Badyaev, A.V. & Hill, G.E. 2000. Evolution of sexual dichromatism: contribution of carotenoid-versus melanin-based colouration. Biological Journal of the Linnean Society 69: 153-172 VIEW
Chiale, M.C., Rendón, M.A., Labaude, S., Deville, A.S., Garrido-Fernández, J., Pérez-Gálvez, A., Garrido, A., Rendón-Martos, M., Béchet, A. & Amat, J.A. 2021. The color of Greater Flamingo feathers fades when no cosmetics are applied. Ecology and Evolution 11: 13773-13779 VIEW
Delhey, K., Peters, A., & Kempenaers, B. 2007. . Cosmetic colouration in birds: occurrence, function, and evolution. The American Naturalist 169(S1): S145-S158 VIEW
McGraw, K.J. 2006. Mechanisms of carotenoid-based colouration. In, G.E. Hill & K.J. McGraw (Eds.): Bird Colouration. Volume I. Mechanisms and Measurements, pp. 177-192. Harvard University Press, Cambridge, MS
Image credit
Top right: Greater Flamingo Phoenicopterus roseus © Manuel Díaz Zurita.
If you want to write about your research in #theBOUblog, then please see here.




