Report from a BOU-funded project
LINKED PAPER
31° South: phenotypic flexibility in adaptive thermogenesis among conspecific populations of an arid-endemic bird – from organismal to cellular level. Ângela M. Ribeiro, Clara Prats, Nicholas B. Pattinson, M. Thomas P. Gilbert, Ben Smit. 2018. BioRxiv:461871. VIEW
Cold temperatures have long been recognized as an inescapable physiological stressor for small-bodied endotherms such as passerine birds. While small birds from north-temperate habitats deal with this challenge through flexibility in thermoregulatory physiological traits there is a paucity of information on whether sub-tropical species use similar mechanisms. Thus, in our recent study (Ribeiro et al. 2018) we combined data from different level of organization (organism- to cellular-level) to provide our contribution to better understand how birds deal with cold in sub-tropical arid-zones.
Background
Arid-zone endemic birds live life on the edge. They face food and water shortages, and cope with burning heat in summer and sub-zero temperatures in winter nights. At cold temperatures birds loose heat from their warm bodies into the environment. To counterbalance this heat loss and ensure functionality of biochemical networks and organism physiological responses, birds need to actively warm up. This is achieved by burning cellular fuels to power contraction in skeletal muscle. At the organism-level, this response is quantified as the maximum thermoregulatory metabolic capacity (Msum).
Evidence shows that increases in Msum is critical for overwinter survival [1] [2] and several mechanisms, from whole-organism level (increase of overall body condition and hypertrophy of thermogenic muscles; [3] [4]) down to the biochemical level (increase activity in oxidative metabolism in mitochondria; [5] [6]), have been proposed. Nevertheless, support for those mechanisms comes primarily from passerines living in north-temperate ecosystems, where primary productivity is high and therefore individuals can afford the large energetic cost of producing heat. Hence a question is begging: how are arid-zone endemic birds solving the challenge of keeping warm despite low energy resources? Do they use the same strategies?
Approach, results and implications
To improve our understanding of the physiological underpinnings of adaptive thermogenesis in arid ecosystems we studied a small passerine (Karoo Scrub-robin Cercotrichas coryphaeus) living in the subtropical arid-zone of southwestern Africa. This bird lives an area of low primary productivity and spans a thermal gradient from west (mean minimum temperature in winter = 8 °C) to east (sub-zero minimum temperature in winter).
We hypothesized that birds from populations experiencing sub-zero winter temperatures would exhibit more pronounced increase of Msum than populations from milder conditions. Moreover, we anticipated that this high metabolic capacity at organismal level would stem from increased cellular aerobic metabolism (increased density of mitochondria and lipid droplets), rather than muscle hypertrophy simply because it may not be affordable given the low and sparse energy resources.
To test our hypothesis we developed an integrative approach encompassing several levels of organization (Figure 1). We measured Msum, body condition, mass of thermogenic muscles and two indices of cellular aerobic capacity from populations living in three environmentally different regions.
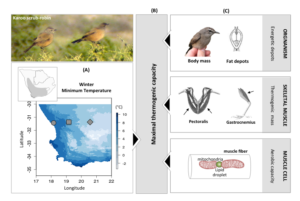 Figure 1. Framework developed by Ribeiro et al. (2018) to study the mechanisms of adaptive thermogenesis in the arid-endemic Karoo scrub-robin. (A) The role of environmental variables in shaping (B) maximal thermogenic capacity and (C) the underlying mechanisms at several levels of organization. Karoo scrub-robin range and study sites are depicted in the maps.
Figure 1. Framework developed by Ribeiro et al. (2018) to study the mechanisms of adaptive thermogenesis in the arid-endemic Karoo scrub-robin. (A) The role of environmental variables in shaping (B) maximal thermogenic capacity and (C) the underlying mechanisms at several levels of organization. Karoo scrub-robin range and study sites are depicted in the maps.
We found that i) the mass of thermogenic muscles remained unchanged regardless of minimum temperature, ii) the density of mitochondria and lipid droplets was maintained seasonally in pectoral muscles (Figure 2); iii) Msum was seasonally flexible, but not increasing with lower temperatures (Figure 3A); iv) the rate of heat loss remained unchanged in the population experiencing the lowest Tmin; v) cold limit (temperature at which birds reached their maximum metabolic capacity) decreased with decreasing minimum temperature (Figure 3B).
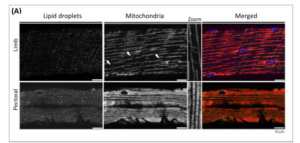 Figure 2. Example of confocal micrographs depicting mitochondria and lipid droplets used to estimate mitochondria and lipid droplets densities, a proxy of cellular aerobic capacity. Merged image show mitochondria (red), lipid droplets (green) and nuclei (blue).
Figure 2. Example of confocal micrographs depicting mitochondria and lipid droplets used to estimate mitochondria and lipid droplets densities, a proxy of cellular aerobic capacity. Merged image show mitochondria (red), lipid droplets (green) and nuclei (blue).
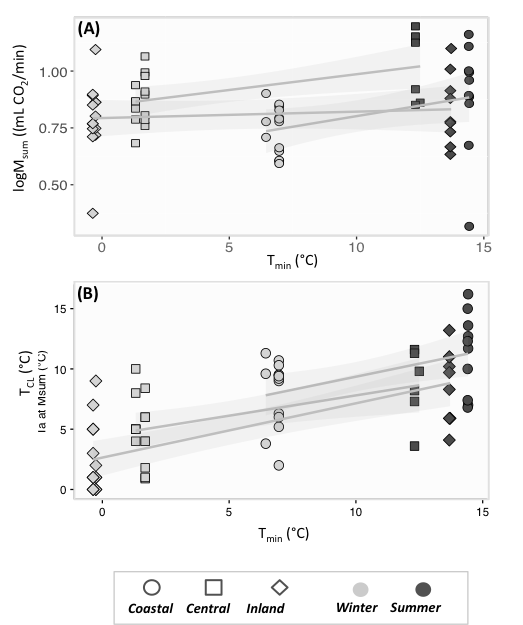
Figure 3. Variation in physiological traits in response to mean minimum temperature: (A) Maximum thermogenic metabolic capacity (Msum), and (B) cold limit (TCL).
Collectively, our results indicate that at least this arid-zone sub-tropical small bird species does not conform to the cold-adaptation hypothesis [2] corollary that winter temperature selects for increased thermogenic performance or the climate variability hypothesis [7] [8] that predicts wider thermal tolerances for organisms experiencing broader climatic fluctuations. We therefore put forward that it is flexibility in cold sensation and not in high Msum that might be the critical mechanism for successful overwintering in the cold winters of the arid sub-tropical habitats. An understanding of the sensory perception of cold in Karoo scrub-robin, as the first system to mediate organism-environment interactions is certainly warranted.
Funding
This study was funded by a Marie Sklodowska-Curie Individual fellowship (European Union Horizon 2020 Research and Innovation Programme, grant 655150 – BARREN) and a British Ornithologists’ Union small research grant.
Nominate this article for a BOU Science Communication Award.
References
- Marsh, R.L., Dawson, W.R. 1989. Avian Adjustments to Cold. Advances in Comparative and Environmental Physiology, vol. 4, Berlin, Heidelberg: Springer Berlin Heidelberg; pp. 205–53. doi:10.1007/978-3-642-74078-7_6.
- Swanson, D.L., Garland, T. Jr. 2009. The evolution of high summit metabolism and cold tolerance in birds and its impact on present-day distributions. Evolution 63:184–94. doi:10.1111/j.1558-5646.2008.00522.x.
- Vézina, F., Jalvingh, K.M., Dekinga, A, Piersma, T. 2006. Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Exp Biol 209: 3141–54. doi:10.1242/jeb.02338.
- Zheng, W-H., Liu, J-S., Swanson, D.L. 2014. Seasonal phenotypic flexibility of body mass, organ masses, and tissue oxidative capacity and their relationship to resting metabolic rate in Chinese bulbuls. Physiol Biochem Zool 87:432–44. doi:10.1086/675439.
- Dawson, W.R., Carey, C., Vanthof, T.J. 1992. Metabolic Aspects of Shivering Thermogenesis in Passerines During Winter. Ornis Scandinavica 23: 381–7.
- Likes, E.T., Swanson, D.L. 2011. Phenotypic flexibility in passerine birds: Seasonal variation of aerobic enzyme activities in skeletal muscle. Journal of Thermal Biology 36: 430–6. doi:10.1016/j.jtherbio.2011.07.011.
- Jansen, D.H. 1967. Why mountain passes are higher in the tropics. The American Naturalist 101: 233–49. doi:10.1086/282487.
- Bozinovic, F., Naya, D.E. 2014. Linking Physiology, Climate, and Species Distributional Ranges. In: Martin LB, Ghalambor CK, Woods HA, editors. Integrative Organismal Biology, Wiley-Blackwell; pp. 277–90. doi:10.1002/9781118398814.ch17.
Image credits
Featured image: Karoo Scrub-robin Cercotrichas coryphaeus | Alandmanson | CC BY SA 4.0




